Request a qoute.

Energy-efficient treatment of odorous compounds
Odorous compounds such as VOCs (Volatile Organic Compounds), H2S and Volatile Fatty Acids (VFAs), can be found in kitchen ventilation and a wide array of different industries. Although the odors do not necessarily impose physical health problems, malodors disturb the surrounding, resulting in complaints towards the source.
Odorous VOCs can originate from microbial or thermal decomposition of organic matter, which is a common process in breweries, wastewater treatment plants, cooking and food industries.
The sources of H2S emission are similar to the ones of VOCs, with the main difference of the presence of sulfur compounds in the source substrate. For example, the organic material in food industries and wastewater treatment plants contains a significant concentration of sulfur compounds, leading to H2S emissions. H2S is also common in breweries, biogas production plants, due to the anaerobic reduction of sulfur compounds in the bioreactors or digesters.
The human nose and odor perception
The human nose has been evolving over the millennials developing different sensitivities for different compounds. For instance, the nose has a very high sensitivity towards of compounds emitted from decomposing organic matter. In this way, the ingestion of rotting food could be avoided during the course of the history, preventing the associated diseases. Rotting food emits sulfur compounds such as H2S and VOCs such as aldehydes and hexyl acetate. Therefore, it is not surprising that the human nose can detect trace concentrations of these compounds as shown in the table below. On the other hand, the nose is less sensitive to compounds not emitted naturally such as toluene.

Examples of odorous compounds, character and odor threshold in ppm
| Compound | Character | Odor Threshold [ppm] |
|---|---|---|
| Methyl mercaptan | Decayed cabbage, garlic | 0.002 |
| H2S | Rotten egg | 0.01 |
| Acetaldehyde | Fruity | 0.05 |
| Hexyl acetate | Fruity green apple or banana sweet | 0.12 |
| Formaldehyde | Acrid, suffocating | 0.80 |
| Toluene | Sweet, pungent | 2.90 |
As shown by the table above, the concentration at which odors are a problem is in the order of magnitude of parts-per-million (ppm), and in some cases even lower. Therefore, odor emissions are particularly difficult to treat, as the purification system needs to have a very high performance. Furthermore, when the odor is emitted in an open space, the issue is more complex, since the odor values are affected by external agents such as atmospheric conditions.
We offer many types of air analyses aimed at identifying the odor levels. Read more about Air and odor analysis.
Mellifiq’s solutions for odor removal
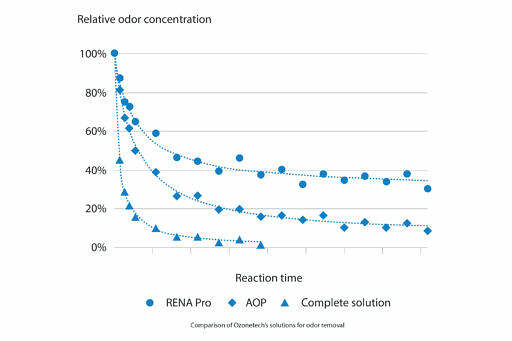
Mellifiq has developed several solutions for solving odor problems in many applications. Based on the case situation and the requirements from the customers, we select and design the most efficient solution for removing unpleasant odor. Each solution can be composed either by one single ozone stage or by a multi-stage system. An overview of the performance of Mellifiq solutions for odor removal is shown in the figure.
Single-stage ozone solution – RENA Pro
In many cases, a single-stage ozone solution already has a great reduction of the odor emissions, as shown by the RENA Pro curve in the figure above. Since ozone is a powerful oxidation media, it quickly reacts with the odorous molecules. These molecules are efficiently oxidized, leading to the formation of carbon dioxide and water, completely odorless and harmless compounds. This oxidation mechanism is valid both for odors from organic compounds, such as VOCs, and from sulfur compounds, such as H2S, as shown.
The only difference is the formation of sulfur dioxide (SO2) instead of carbon dioxide (CO2) when H2S is removed. However, the final result in terms of odor removal is the same, since the odor threshold of SO2 is 1000 higher than the one of H2S. This means that the odor from SO2 is not significant compared to the one from H2S.

Multi-stage solution – AOP
If the requirements for odor removal are particularly stringent, an additional stage can be combined to the RENA Pro, creating an AOP (Advanced Oxidation Process) solution. An Saniray UV reactor is often implemented as additional stage, since the UV light reacts with ozone, creating hydroxyl radicals. These radicals are even more reactive than ozone, attacking the odorants more aggressively. Having radicals involved in the reaction process results in a chain-reaction mechanism, where one single radical can oxidize a high number of odorants.
Such reactive conditions are typical of the high temperatures reached in thermal incinerators. However, due to synergy between ozone and UV, we can mimic the same reactivity at room temperature, greatly decreasing the operational costs for the odor treatment.
Read more on our UV systems.