A powerful oxidant
Ozone is a very powerful oxidation agent. It is easily soluble in water and its ability to eliminate the microorganisms that form pollutants is very good. Once the problem is solved, the ozone dissolves – our method forces the ozone to react with the pollutants and break them down, after which any residual ozone returns to oxygen.
| Chemical formula | O3 |
|---|---|
| Molecular weight | 47.998 g/mol |
| Density, gas (0°C, 101.3 kPa) | 2.144 kg/m3 |
| Melting point (101.3 kPa) | -192.5°C |
| Boiling point (101.3 kPa) | -111.9°C |
| EU hazard classification | Oxidant (O) |
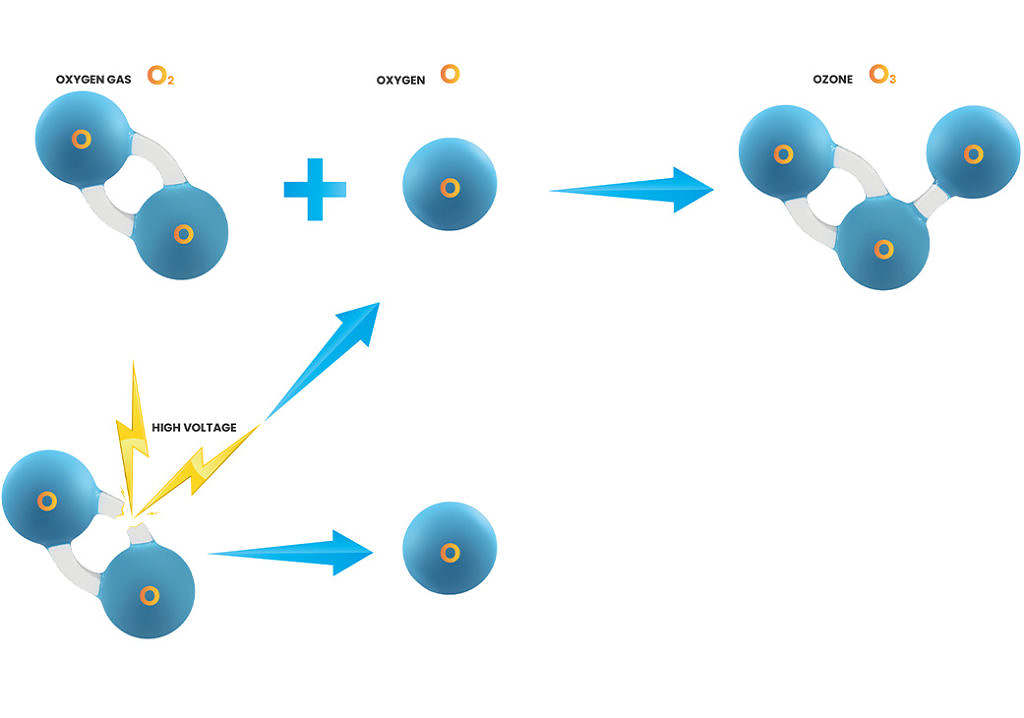
The chemistry of ozone formation
Ozone can be produced photochemically with UV light or with electrical discharges (corona discharges) in an oxygen-filled atmosphere. Thanks to our own patented technique, our equipment uses the advantages of corona discharge to the maximum. Air or oxygen is channelled between two electrodes and then subjected to electronic discharges. The oxygen atoms are then partly atomized and form ozone when free oxygen molecules react with the oxygen molecules present.
The electrons in the discharge supply energy to dissociate the atoms in the oxygen molecule:
O2 + e– => O + O
These free atoms react directly with each other or with oxygen molecules to form ozone:
O2 + O => O3
Limit values
The Swedish National Board of Occupational Safety and Health has drawn up hygienic limit values (acceptable average levels in inhaled air) for ozone. Two main levels are used:
- Level limit value (LLV): Highest acceptable average content in inhaled air during 8 hours
- Ceiling limit value (CLV): Highest acceptable average content in inhaled air during 15 minutes
For ozone the threshold limit value is 0.1 ppm (0.2 mg/m3) and the ceiling is 0.3 ppm (0.6 mg/m3) (AFS 2015:7). According to the Board, ozone is not carcinogenic or allergenic and does not impair reproduction; nor is it easily absorbed by the skin.
Ozone levels above 0.3 ppm irritate the respiratory tract and the mucous membranes of the eye, while levels around 100 ppm are dangerously toxic within a few minutes.
Links: Ozone (Wikipedia), Swedish National Board of Occupational Safety and Health, AFS 2018:1