Request a qoute.
Innovative and efficient water treatment in the pharmaceutical industry
The global use of pharmaceuticals has been growing steadly for the past decades and the overall spending is predicted to continue grow in the coming years. This rise is driven by a range of factors including the ever growing globlal competition offset by expiration of patents and the rise of generics, new product intakes to mention a few. All of this has resulted in increasing concentrations of pharmaceutical residues in our wastewater streams. This presents a huge struggle for wastewater management around the world to address the potential impacts of these pharmaceutical residues on drinking water quality, the ecosystem and the health of humans and other living organisms.
Current treatment technologies used in wastewater treatment plants are usually not fit to remove microbial stable chemical pollutants and the evaluation of the removal efficiency applied today, is not complete. Pharmaceutical residues and other emerging substances pass through modern wastewater treatment plants and end up in the receiving waters and sludge.
Substances such as antibiotics (e.g. Amoxicillin and Ciprofloxacin), beta blockers and drugs like morphine and codeine are difficult and often impossible to treat in wastewater treatment plants due to their complex chemical structures. Such substances have an adverse effect on fish, plants and whole ecosystems and accumulate over time due to their non-biodegradable characteristics.
Mellifiq offer more than 20 years of experience implementing advanced oxidation solutions and technologies in water treatment for a wide range of industies and municipalites and governmental organisations. Our innovative solutions, combining ozone and conventional technologies, are ideal for water and air treatment in the production process as well as treatment of the discharged wastewater.
Our goal is to offer a more efficient and sustainable solution that helps you improve your business profitability and output quality. By reducing your energy costs you will also automaticaly lower your production facilitie’s environmental impact.
A health risk that can be eliminated
Can pharmaceutical residues be removed effectively and sustainable?
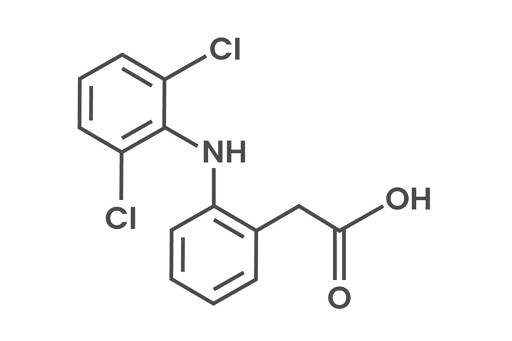
Diclofenac
Prozac
Naproxen
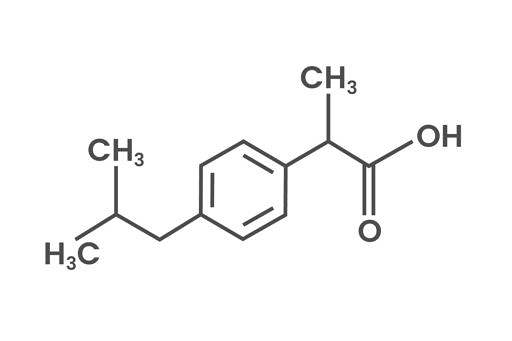
Ibuprofen
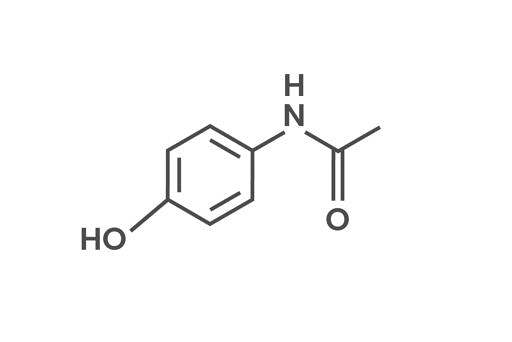
Acetaminophen
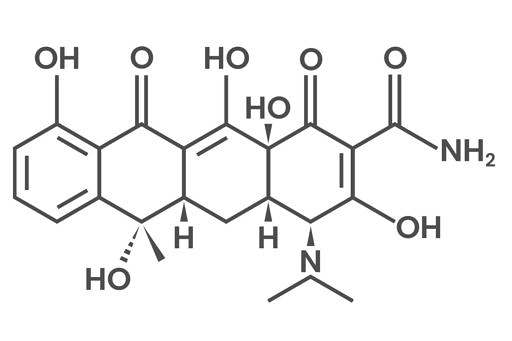
Tetracycline
Yes, they can
Medicines such as antibiotics, pain killers, and antiseptics used to sustain health also have detrimental, environmental side effects.
A large portion of consumed drugs has inevitably affected our environment for a long period of time. It is now possible to end the accumulation of micropollutants through effective removal of active pharmaceutical ingredients (API).
In parallel worldwide private and public initiatives are taking action to encourage the current development in neutralizing harmful micropollutant emissions.
Mellifiq is a global engineering company, specializing in implementing the most efficient treatment methods for API removal for municipal wastewater treatment plants and the pharmaceutical industry.
We present our technology, engineering expertise, and consulting services required in order to deliver the world’s most cost-effective and energy-efficient solution.
Only a fraction of active pharmaceutical ingredients (API) can be removed with traditional technologies, leading to harmful effects on flora, fauna and ultimately human beings.
Commonly found pharmaceutical residues
Overview of commonly found active micropollutants in wastewater effluents:
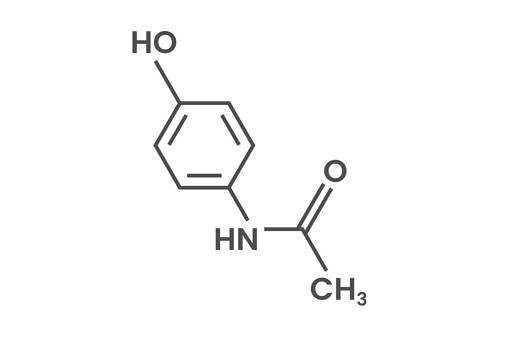
Paracetamol
Paracetamol (N-acetyl-4-aminophenol) is a group of medicines (e.g. Alvedon) found in mild analgesics or non-steroidal antiinflammatory drugs that are sold in large quantities. They are commonly used for reduction of pain and fever symptoms. The projected annual world production of paracetamol is about 145,000 tons. The paracetamol molecule consists of a benzene ring core, substituted by one hydroxyl group and the nitrogen atom of an acetamide. More info about the paracetamol degradation with ozone treatment can be found below.
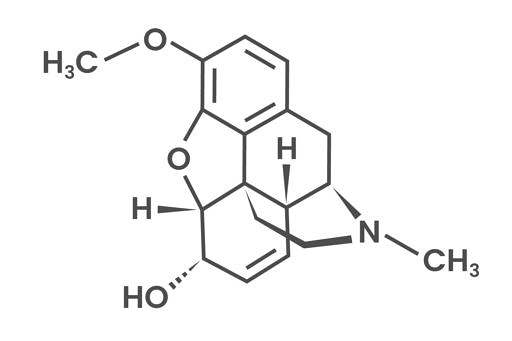
Codeine
Codeine (3-Methylmorphine) is a narcotic medication (e.g. Oramorph) used to treat moderate pain and cough. It consists of an aromatic ring and a quaternary carbon atom linked to a tertiary amine group by two other carbon atoms. This chemical characteristic is also known as the morphine rule. The molecule consists of a total of five rings, out of which three are in the same plane.

Diclofenac
Diclofenac 2-(2, 6-dichloranillino) phenylacetic acid is a non-steroidal anti-inflammatory drug (i.e. Voltaren) that is easily available in medical outlets and hundreds of tons are sold worldwide every year. It consists of two adjacent aromatic rings, with one ring bearing a carboxylate and the other one, a phenyl ring, binds perpendicular to the top of aromatic ring with two ortho-chloro groups.
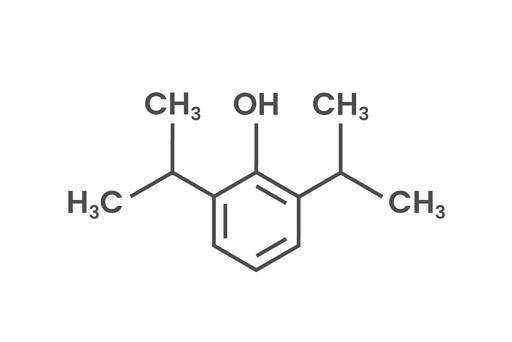
Propofol
Propofol (2,6-Bis (1-methylethyl) phenol) is a stable molecule and an intravenous anesthetic agent used in maintenance of general anesthesia (e.g. Propoven). Propofol consists of a benzene ring and an isopropyl group. Propofol, like the other compounds mentioned above, is effectively degraded to below detection levels with ozone treatment. Propofol in particular has shown a first order reaction in type in a typical wastewater effluent from pharmaceutical industry.
Major sources of pharmaceutical residues
Hospitals
Pharmaceuticals can be found in natural waters as a result of flushing down leftover products at private homes but a large source is hospitals. Therefore ozone technology should be considered to enhance the performance of hospital wastewater treatment plants or as tertiary at municipal wastewater treatment works. It has been found that hospitals contribute to around 20% of the pharmaceutical load in sewer streams as reported by EU The PILLS Project.
Pharmaceutical industry
In the pharmaceutical industry, measures can be taken to eliminate pharmaceutical residues from the production process wastewater. The industry is a major source of pharmaceutical micropollutants which can be identified in municipal wastewater treatment plants. There is an increasing demand on manufacturers to eliminate pharmaceutical residues prior to emitting the process wastewater.
Methods to treat pharmaceutical residues
A number of techniques exist today, which may act as a complement to the secondary, biological treatment found in wastewater treatment plants.
Powdered Activated Carbon (PAC)
PAC acts as an effective micropollutant filter and can be used as a tertiary treatment step in wastewater treatment. It may remove up to 86% of pharmaceutical residues by adsorption and provides not only transformation of target substances but removal.
UV
UV is a cost-efficient way of treating clear wastewater, but has limited ability to break down pharmaceutical residues. It has been reported by Kovalova, et al. (2013) that 33% of micropollutants can be removed on average by applying this technology. UV may effectively remove ICM using high dosages, such as Diatrizoate and Iopamidol.
Ozone treatment
Ozone is a potent oxidizing agent which effectively breaks up the chemical bonds in complex molecules, which makes it an efficient method to remove pharmaceuticals in the wastewater. Ozone can also be used in advanced oxidation processes (AOP) to boost radical formation in addition to the natural formation during ozone reactions. It has been shown that up to 100% of pharmaceutical micropollutants can be removed using ozone. Commonly applied range is 0.3 to 1.2 g O3/g DOC (dissolved organic carbon) according to Barasel, et al. (2015).
Mellifiq has over 20 years experience of advanced oxidation processes and has conducted a large number of projects with 100% removal of pharmaceutical residues in the process water. The figure below displays actual break-down of a common pharmaceutical residue by ozone and ozone-AOP processes.
Destruction of pharmaceuticals with ozone
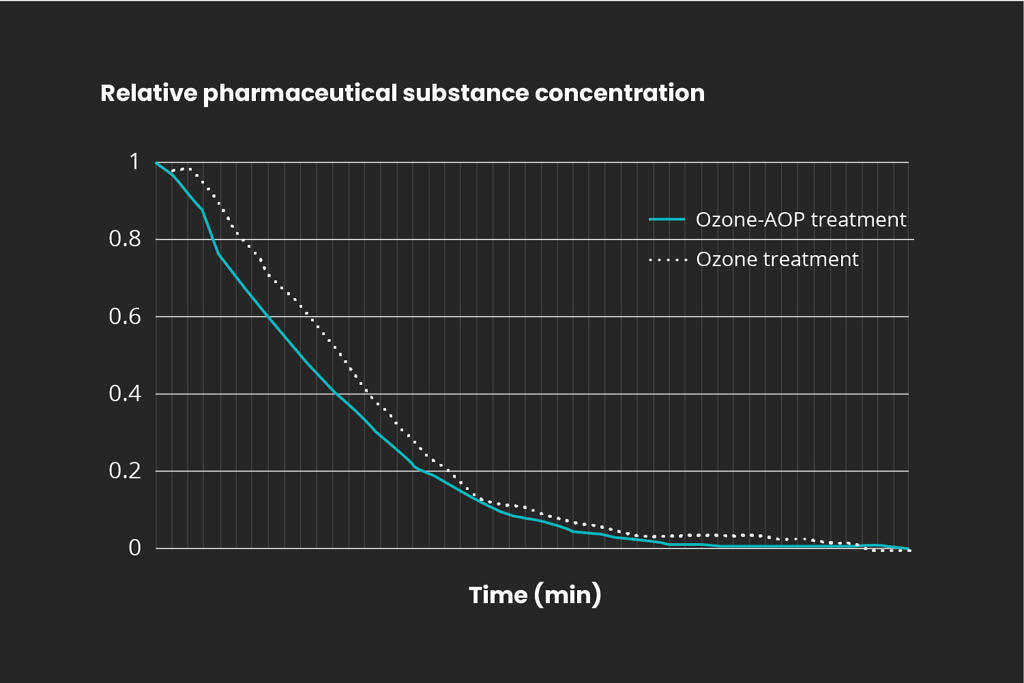

Treatment of pharmaceutical residues with ozone.
About 100,000 diverse chemicals are registered in the Europe Union (EU). Out of these 100,000, about 30,000 are distributed in large quantities, where each one is distributed in quantities of about one ton per year or more for human health consumption. During production, disposal and after regular use, a substantial portion of these active substances (micropollutants) are inevitably entering the aquatic environment.
Pharmaceuticals are one of the most important classes of emerging contaminants in water treatment processes and have a direct impact on human health and the ecosystem. The sources of pharmaceuticals include livestock residues, hospital discharge, aquaculture and release from domestic and industrial wastewater effluents. Among them, treatment of wastewaters has become increasingly difficult due to several reasons, including:
- Discharged wastewater contains clusters of micropollutants (i.e. pharmaceutical active substance) that are resistant to biological wastewater treatment processes. This is due to the complex molecular structures of these compounds.
- Stringent regulations on effluent discharge limits
Untreated, pharmaceutical residues promote the emerging problems of antibiotic resistant bacterial strains.
Treating Micropollutants at Kungsbacka Municipality
Methods for treating pharmaceutical residues at manufacturer: benefits and challenges.
| Method | Benefits | Challenge |
|---|---|---|
| Incineration of process wastewater containing micropollutants | Treatment conducted on-site. No costs for transportation to and handling of third party. | Treatment conducted on-site. No costs for transportation to and handling of third party. |
| Destruction off-site. Transportation to a third-party recycling company. | Scheduled shipping and destruction handled by a third party. | Very high costs. Fees and costs relating to third-party handling. Requires buffer tank and handling of process water between collection of process water. |
| Ozone treatment of pharmaceutical residues | Treatment conducted on-site and on-demand. System footprint less than 4 sqm. Less handling of untreated process water. Very low operational cost. Payback time 0.5 -2 years, depending on flow and substance. Safe use. | New mechanical installation but at a low footprint. |
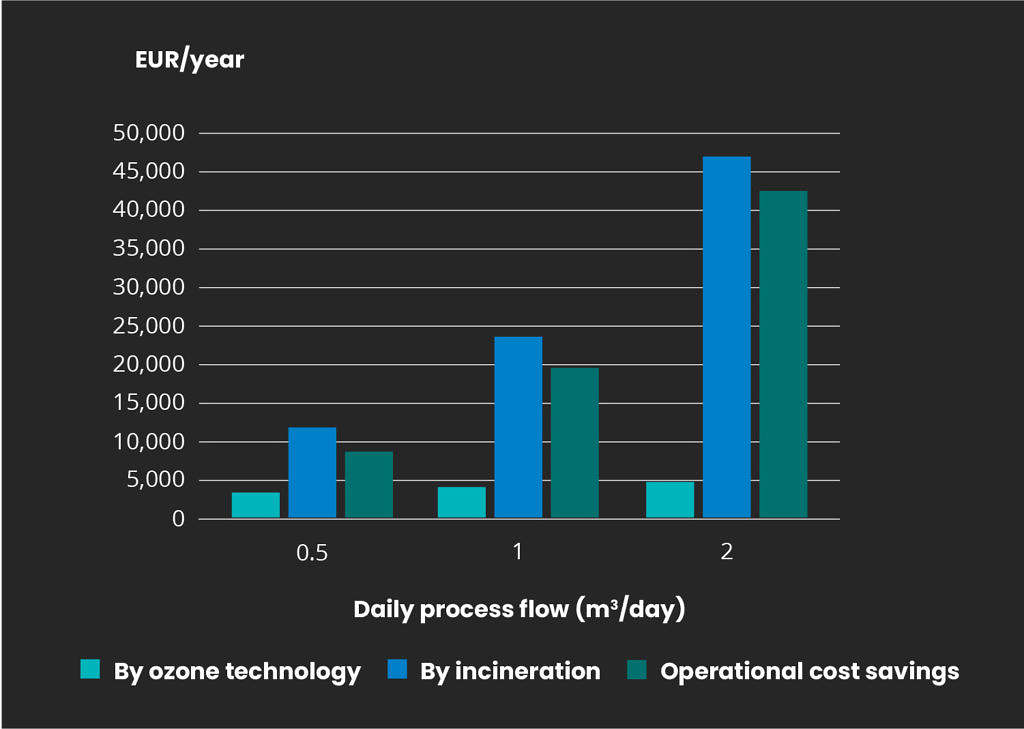
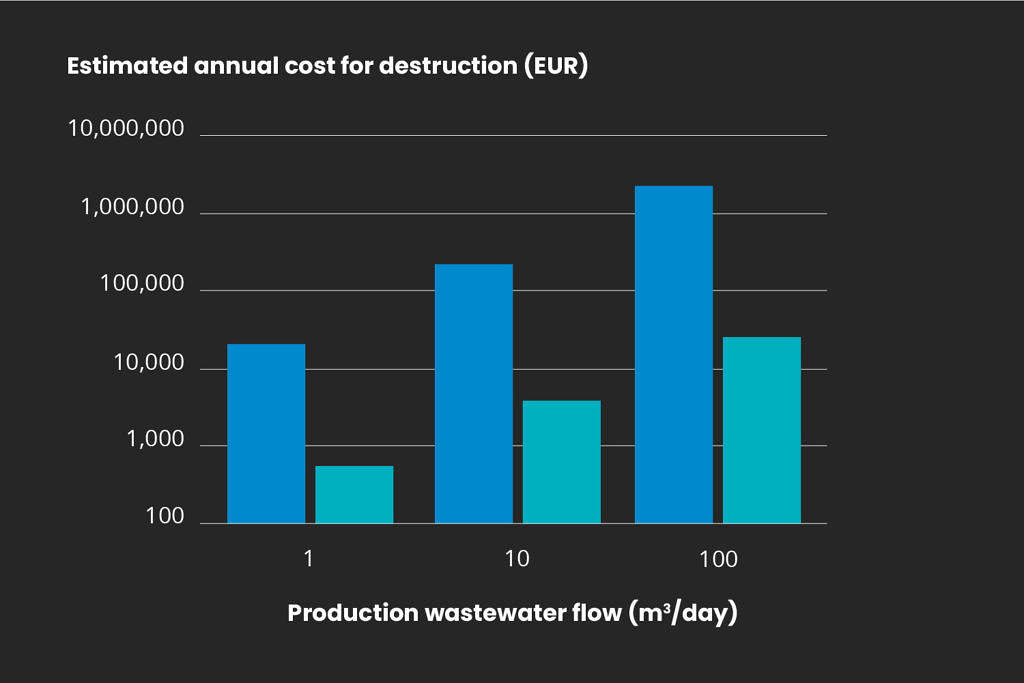
Ozone technology provides a cost-effective alternative to traditional destruction by incineration. Mellifiq employs the Ozonetech RENA CIP or RENA AOP engineered systems to break down these micropollutants which will save large amounts of the operational costs associated with destruction. The figure below displays estimations for operational costs, comparing the heating and evaporating of process water and ozone treatment for micropollutants. Note that any handling costs for off-site incineration destruction are not included in the comparison below.
Annual destruction cost for pharmaceutical residues
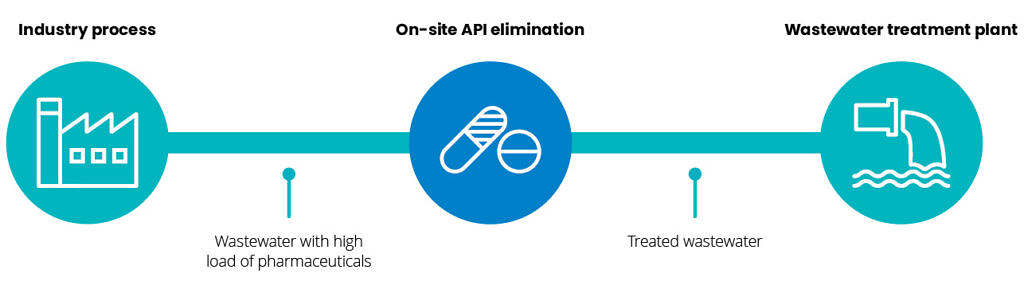
Treat at the source
Large quantities of micropollutants are released directly from hospitals and pharmaceutical production plants in high concentrations. Still the majority of all API residues can be traced to municipal wastewater treatment plants.

Pharmaceutical residues removal system
At pharmaceutical production facilities
At hospitals and healthcare facilities
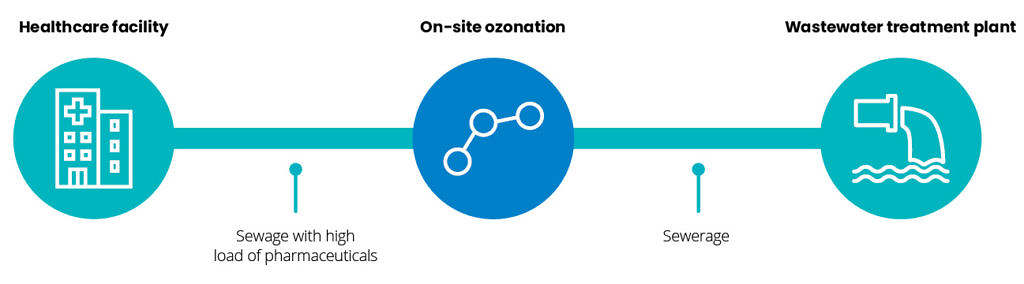
At municipal wastewater treatment plants

Choose proper service, scope and capacity.
A successful full-scale advanced treatment system involves proper analysis, technology and services from start to finish. Mellifiq is a solution provider, supplying oxidation technologies and separation systems, and strongly dedicated to finding the suitable combination of technologies tailored to each facility.
In-house engineering services
Each and every system leaving our production facility has gone through the same design steps. All steps are carried out by our in-house experts.
Pilot projects
This unique service enables complete customization based on the specific water quality.
Installation
We offer complete installation services when needed and full assistance throughout the process.
Commissioning
When the system has been installed, we optimize the operation based on local site conditions for a smooth operating experience in order to reach maximum efficiency and the lowest possible operating costs.
Manufacturing and quality control
The designed system is constructed and assembled by Ozonetech. As an original equipment manufacturer, we validate performance and operation before shipping.
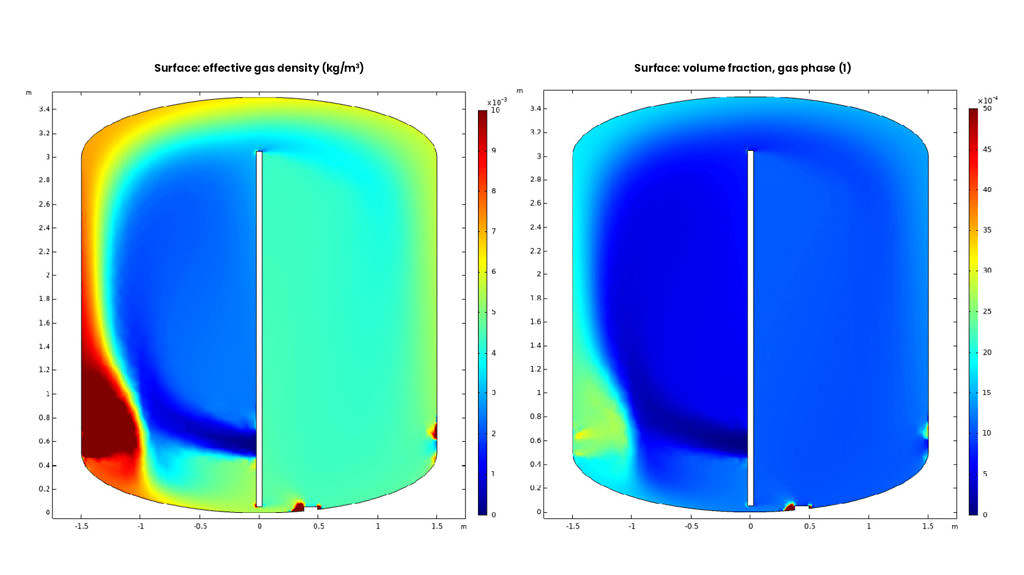
CFD
We use computational fluid dynamics to ensure the maximum treatment capacity of oxidation technology.
System design
We customize the complete solution using a number of technologies such as ozone, carbon filtration and advanced oxidation processes (AOP), fine-tuned with CAD.
Pitfalls. Be aware of design flaws. Here is a quick guide how to avoid them..
- Perform a proper analysis of API concentrations and water quality to determine the dissolved ozone concentration and type of system.
- Make a careful choice of suitable pretreatment and post-oxidation treatment methods to avoid inadequate results or excessive maintenance.
- Choose the proper combination technology to avoid high investment and operating costs.
- Choose a turnkey solution from a supplier with extensive engineering and supply expertise.


Superior corona discharge ozone systems.
We base each micropollutant removal solution on tailored design and engineering, combining ozone with suitable pre-treatment and polishing steps. Depending on the requirements, we design our systems with up to 100% removal of pharmaceutical residues.
All Ozonetech RENA systems are designed with utmost attention to detail and performance, specifically low energy consumption and low maintenance need. Pharmaceutical residues are typically present at nanogram and microgram levels depending on the source. Our most common designs cover ozone treatment, which degrade micropollutants at a very high rate, followed by carbon filter polishing, or extended ozonation. Our designs allow for treatment costs of less than €0.0065/m3.

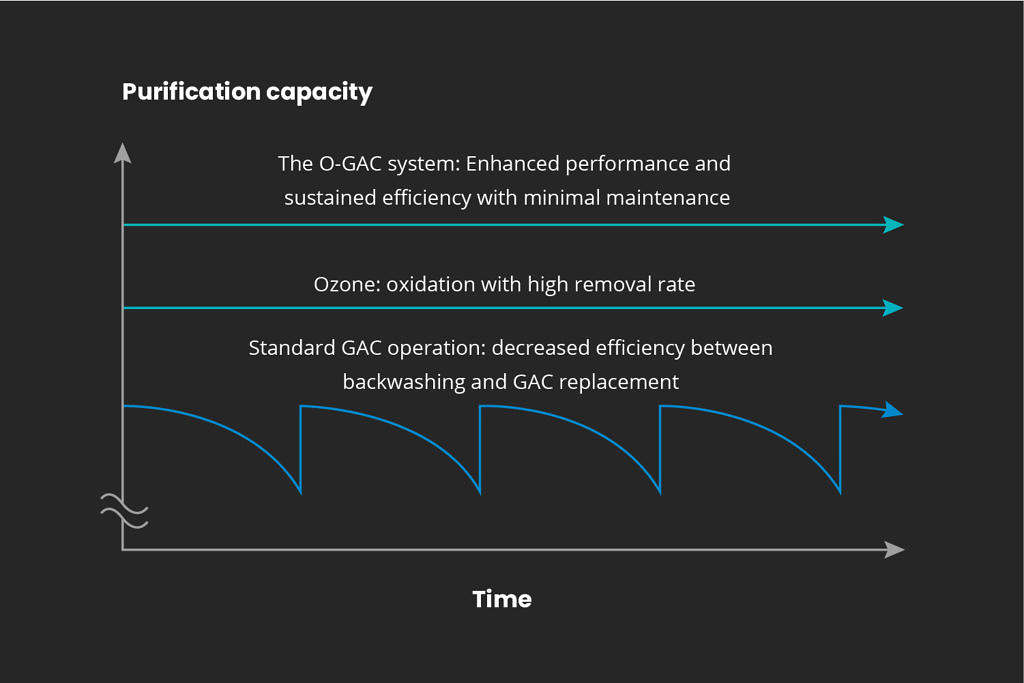
In-house designed final polishing.
All water treatment solutions combining a RENA system and a final polishing step are sized and configured for each specific client and task. With the introduction of Water Maid, a Mellifiq brand, O-GAC™, time and cost of replacing the activated carbon is highly reduced.
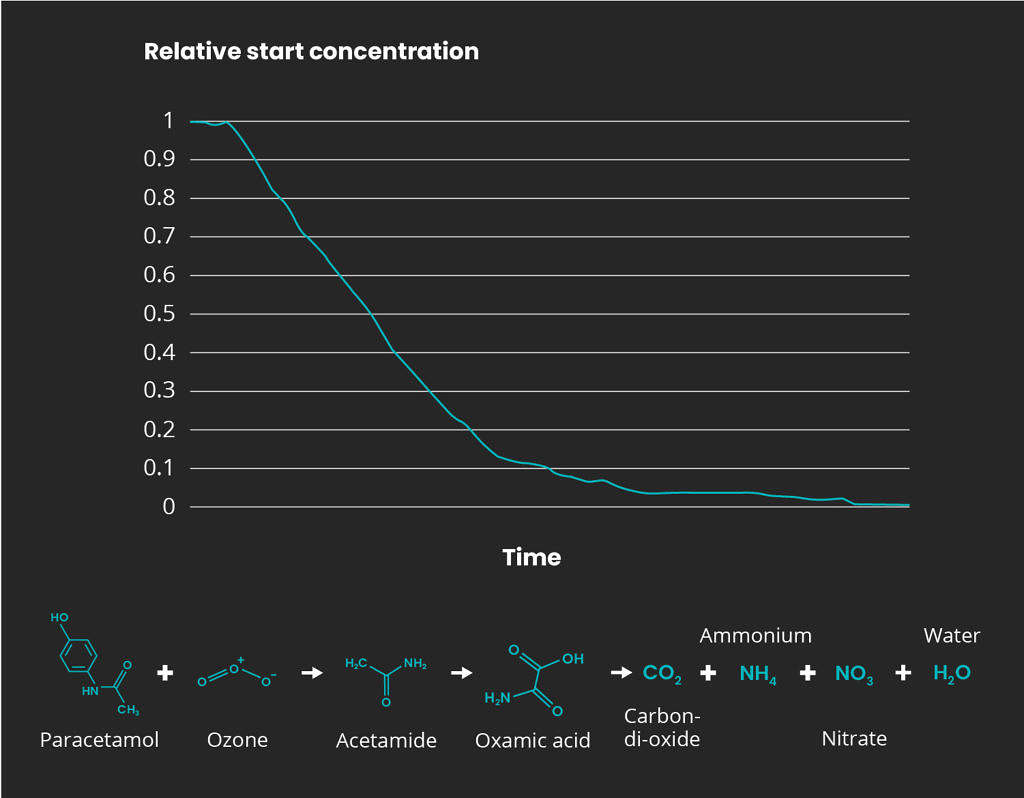
Paracetamol degradation example
In-house designed pilot-scale tests demonstrated › 99 % removal of paracetamol directly from a pharmaceutical production plant effluent with optimum concentration of ozone dose. One pathway for paracetamol degradation during the treatment process with ozone is hydroxyl radical attack on the aromatic ring with subsequent hydroxylation. Pharmaceutical compounds has a reaction rate that is directly dependent on the concentration of active substance present in the mixture. A schematic figure is presented below where the oxidation pathway and a reaction order representation is displayed.
Ozone treatment of paracetamol
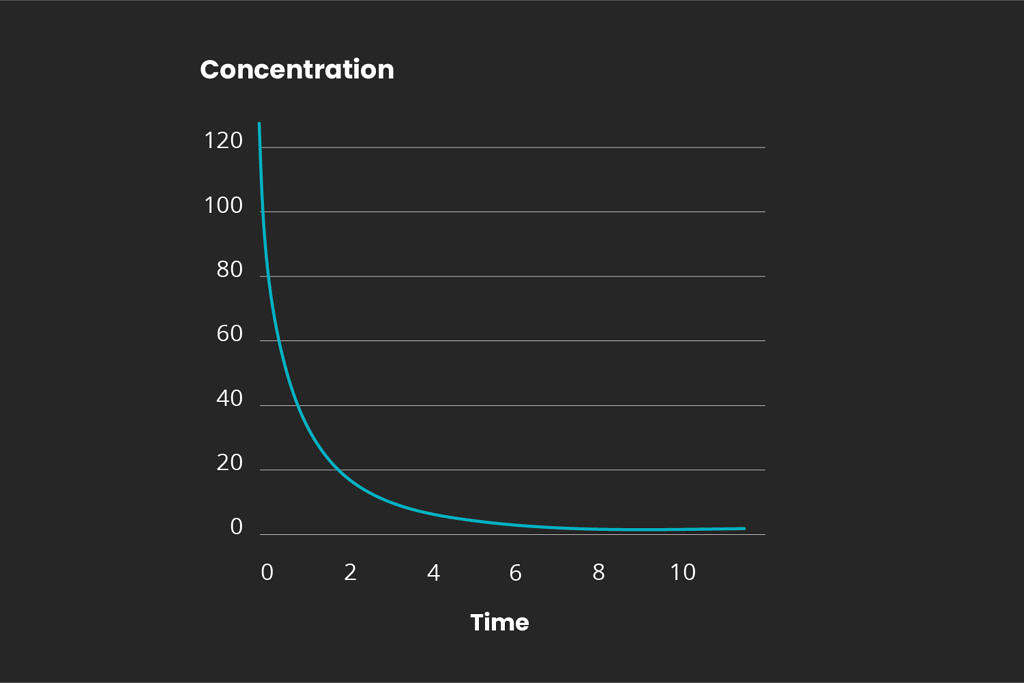
Overview of reaction order
There are different kinds of chemical reactions. The order for any chemical reaction is defined as the reaction rate dependency of the concentration of the reactants. Representative graphs for the 1st, 2nd, and 3rd reaction orders are displayed below:
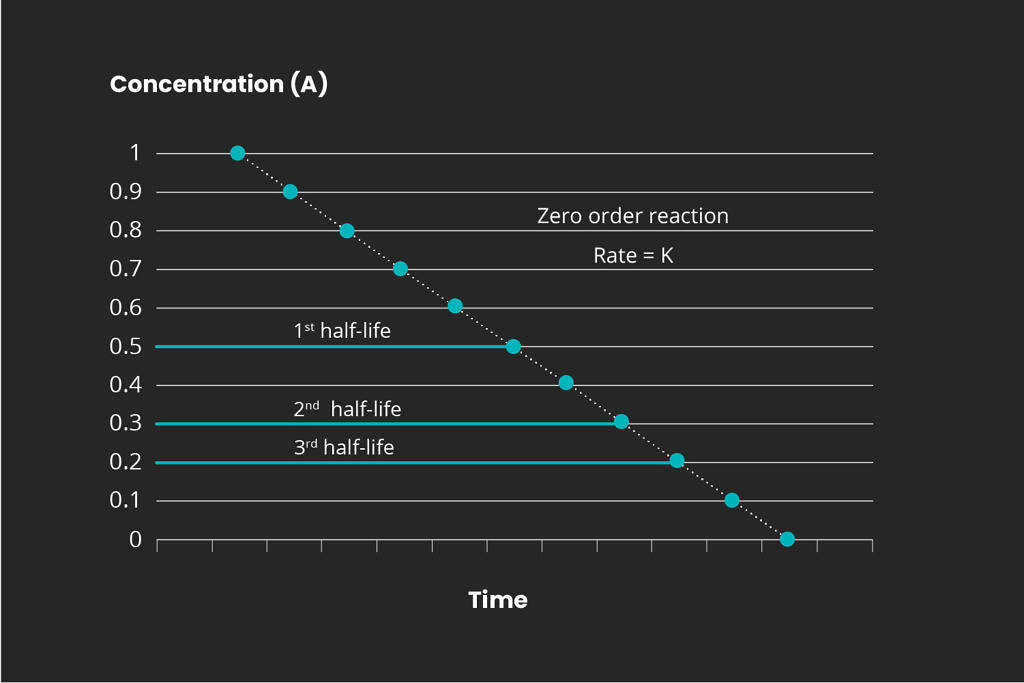
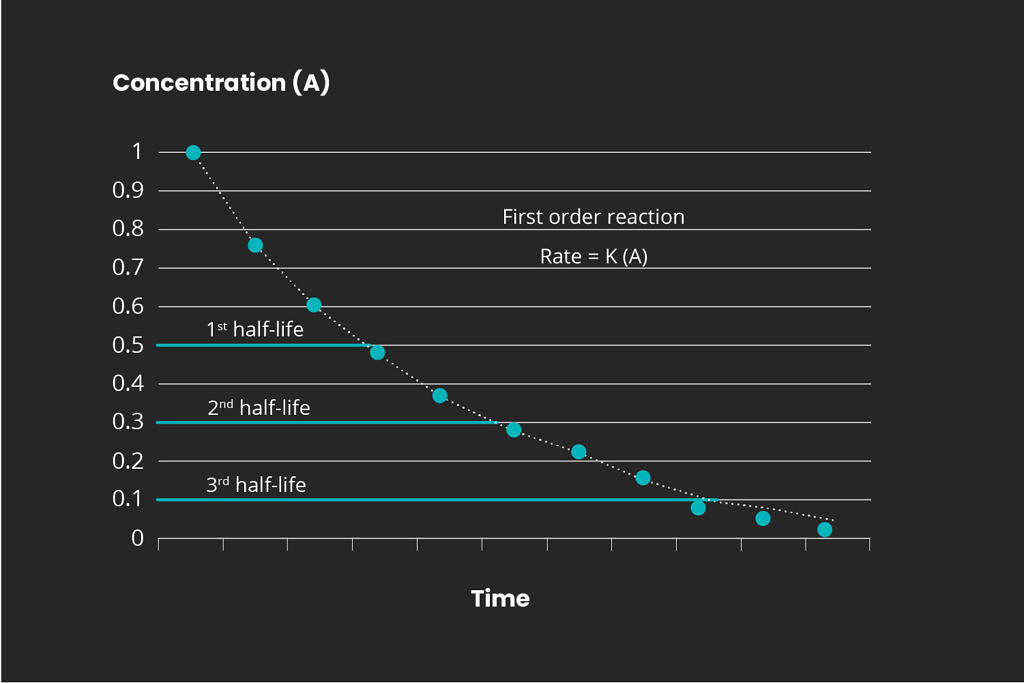
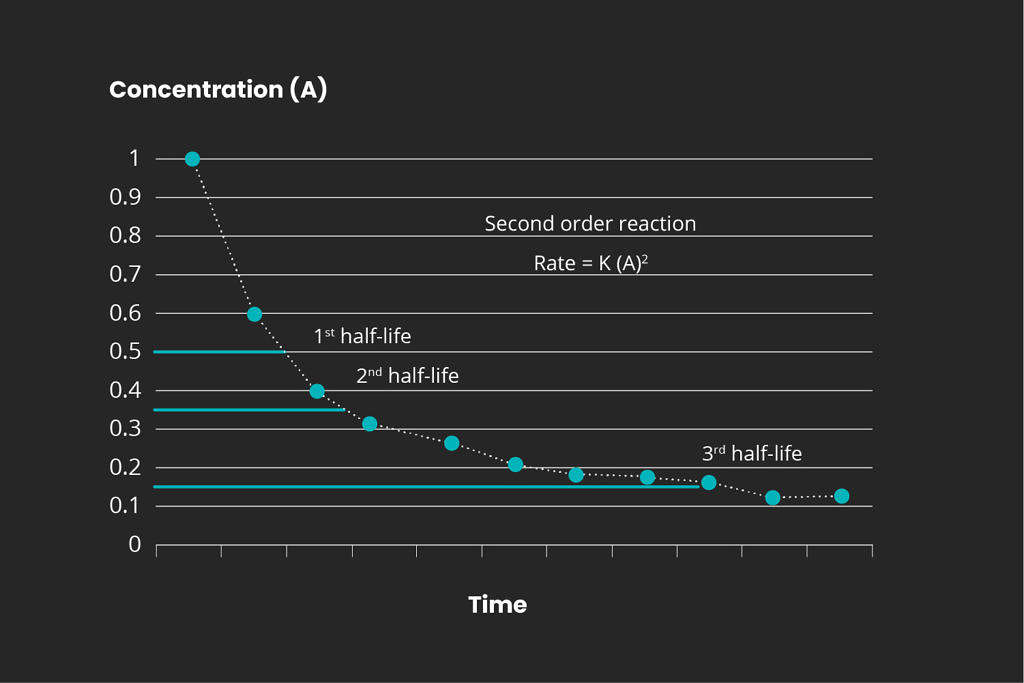
It is important to determine the reaction kinetics in order to scale a full-scale ozone treatment system. Mellifiq offers pilot project services to determine the specific treatment requirements for specific wastewater streams, including pharmaceutical plants and hospital wastewater. Contact us for more information.
Recommended products
Water Maid
- Pressurized multimedia and adsorption systems
- Automatic backwashing
- Wide range capacity
- Highly efficient polishing for complete removal

Related reference project.


